Menu
Services
1) GXP Auditing
1) GxP auditing for Supplier Qualification, Re-qualification and surveillance as per various Regulatory and Statutory requirements. We conduct GMP audits/ inspections to verify the compliance status of the manufacturer and suggest further improvements to comply with regulatory requirements. The objective is also to monitor the effectiveness of GMP implementation programmes. All information shall be treated as confidential and the mechanics of operation shall be based on transparency and ethical principles.
This helps the firm in assuring consistent quality and adherence to cGMP across the entire supply chain i.e.; from procurement to distribution, complaint evaluation and Management quality reviews. We also carry out audits to assist the firms in establishing feasibility of third party manufacturers, qualifying vendors of raw materials, primary packaging material, sterilization facilities, CMOs etc. We conduct detailed audits w.r.t. applicable regulatory requirements as mentioned below:-

- Auditing of API manufacturing sites.
- Auditing of Excipient manufacturing sites.
- Auditing of Packaging Material manufacturing sites.
- Auditing of Gamma and ETO sterilization facilities.
- Auditing of formulation facilities (Injectables, Ophthalmic, Liquids and Orals etc.).
- Preparation and surveillance audits for ISO 9001,14001, 45001, 22000, 17025 and 13485 certifications.
- Auditing of contact testing labs as per 17025 and other applicable requirements.
- Auditing of contract Development organizations.
- Auditing of clinical research organizations.
- Auditing of Contract manufacturing Organizations.
2) Management of complete supplier portfolio for client/customer organizations
- Support in preparation of supplier qualification program.
- Support in preparation of annual audit schedules.
- Execution of supplier audits as per schedule.
- Support in preparation of supplier risk assessments.
- Support in Annual evaluation of suppliers.
- Support in performance of supplier investigation/for cause audits.


3) Management of self inspection program for client/customer organizations
- Support in preparation of annual self inspection plans.
- Training of Internal auditors.
- Performance of internal audits as per defined schedules.
- Submission of internal audit reports and CAPA support.
- Corrective action & Preventive actions (CAPA) effectiveness check.
- Monitoring of continuous improvements in Quality Management system.
- Support in Management Quality Reviews.
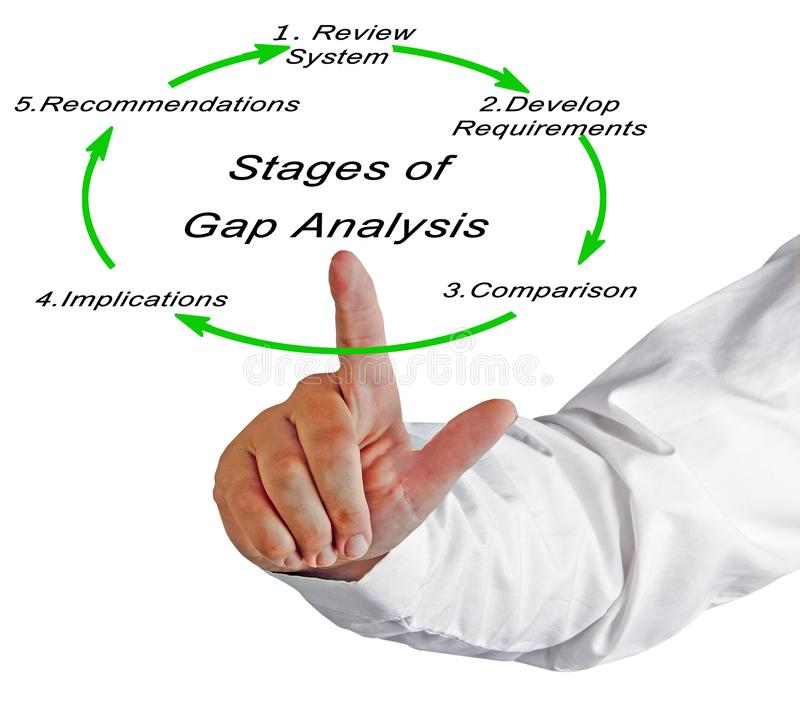
4) Gap Assessment against Regulatory requirements such as USFDA, EUGMP, PICS, TGA, Eurasia etc.
- Assessment of existing Quality Systems against the Regulatory requirements.
- Evaluation of Gaps.
- CAPA proposals.
- CAPA implementations.
- Effectiveness checks.
- Preparation and assessments of manufacturing sites for Regulatory Audits.

5) GMP Trainings
- Support in preparation of GMP Training schedules.
- Train the Trainer Trainings.
- Performance of GMP trainings as per regulatory requirements.
6) Green Field project consultations
- Consultation support in green field projects to comply with various regulatory requirements.
- Support in clean room developments.


7) Validation services
- Support in validation and qualification services of HVAC systems.
- Support in validation and qualification services of utilities such water systems, nitrogen systems, air compressors etc.
- Support in validations of autoclaves, Dry Heat sterilizers and ETO sterilizers.
- Support in review of process validation/Revalidation and Equipment Qualification/Requalification Protocols/reports.
8) Computer System Validation (CSV) Services
- Assessment of existing systems for CSV requirements.
- Support in development of plans for CSV.
- Performance of CSV as per approved plans.
- Verifications.


9) Calibration services from ISO 17025 accredited lab
- Calibration services for all kind of instruments from ISO 17025 accredited lab.
- Auditing of calibration laboratories.
10) Procurement services for QC instruments , Utility Equipments and Production Equipments/Instruments
- Provide support in purchase of Laboratory glassware.
- Provide support in procurement of various equipments/instruments required for production, Utility and QC lab.
- Provide support in set up of QC and Microbiology Labs.


11) Support in implementation of Paperless Documentation (eBMR, eQMS and LIMS etc.)
- Assessments of client requirements.
- Implementation of electronic systems in compliance with 21 CFR.
- Verification of systems.
- Audits of electronic systems.
12) Recruitment and Career Counseling Services
- Recruitment support for various technical/Non Technical positions.
- Counseling support to students and industry professionals for their selection and growth in various streams.


13) GMP audit preparation for USFDA, MHRA, PIC/S, ANVISA, WHO-GMP etc.
- GMP gap assessment and remediation of Quality systems, Manufacturing, laboratory and Engineering systems.
- Development of Document Management System.
- Development of entire Quality and GMP System.
- Perform Equipment, Utility and Facility Qualification.
- Perform process, analytical and cleaning validation
- Perform risk assessment of the system and facility.
14) Regulatory support for Dossier Preparation and submission
- Preparation and submission of Dossiers for ROW markets.
- Preparation and submission of Dossiers for US and EU markets.
- Management of Licenses with CDSCO.
- Handling of Regulatory Queries.

GxP auditing
GxP auditing for Supplier Qualification, Requalification and surveillance as per various regulatory and statutory requirements. We conduct GMP audits/ inspections to verify the compliance status of the manufacturer and suggest further improvements to comply with regulatory requirements. The objective is also to monitor the effectiveness of GMP implementation programmes. All information shall be treated as confidential and the mechanics of operation shall be based on transparency and ethical principles.
This helps the firm in assuring consistent quality and adherence to cGMP across the entire supply chain i.e. from procurement to distribution, complaint evaluation and product quality reviews. We also carry out audits to assist the firms in establishing feasibility of third party manufacturers, qualifying vendors of raw materials, primary packaging material, sterilization facilities, CMOs etc. We conduct detailed audits w.r.t.
applicable regulatory requirements as per below:
- Auditing of API manufacturing sites.
- Auditing of Excipient manufacturing sites.
- Auditing of Packaging Material manufacturing sites.
- Auditing of Gamma and ETO sterilization facilities.
- Auditing of formulation facilities (Injectables, Ophthalmic, Liquids and Orals etc.).
- Preparation and surveillance audits for ISO 9001, 14001, 45001, 22000, 17025 and 13485 certifications.
- Auditing of contact testing labs as per 17025 and other applicable requirements.
- Auditing of contract Development organizations.
- Auditing of clinical research organizations.
- Auditing of Contract manufacturing Organizations.


Management of complete supplier portfolio for client/customer organizations:

- Preparation of supplier qualification program.
- Preparation of annual audit schedules.
- Execution of supplier audits as per schedule.
- Preparation of supplier risk assessments.
- Annual evaluation of suppliers.
- Performance of supplier investigation/for cause audits.
Management of self inspection program for client/customer organizations
- Preparation of annual self inspection plans.
- Training of Internal auditors.
- Performance of internal audits as per defined schedules.
- Submission of internal audit reports and CAPA support.
- Corrective action & Preventive actions (CAPA) effectiveness check.
- Monitoring of continuous improvements in Quality Management system.

Gap assessment against regulatory requirements such as USFDA, EUGMP, PICS, TGA, Eurasia etc.

- Assessment of existing Quality systems against the regulatory requirements.
- Evaluation of Gaps.
- CAPA proposals
- CAPA implementations
- Effectiveness checks
- Preparation assessments of sites for Regulatory audits
GMP Trainings
- Preparation of GMP Training schedules.
- Train the Trainer Trainings.
- Performance of GMP trainings as per regulatory requirements.

Green Field project consultations

- Consultation support in green field projects to comply with various regulatory requirements.
- Support in clean room developments.
Validation services
- Support in validation and qualification services of HVAC systems.
- Support in validation and qualification services of utilities such water systems, nitrogen systems, air compressors etc.
- Support in validations of autoclaves, Dry Heat sterilizers and ETO sterilizers.
- Support in review of process validation Protocols/reports.

Computer System Validation(CSV) Services

- Assessment of existing systems for CSV requirements.
- Development of plans for CSV.
- Performance of CSV as per approved plans.
- Verifications.
Calibration services from ISO 17025 accredited lab
- Calibration services for all kind of instruments from ISO 17025 accredited lab.
- Auditing of calibration laboratories.

Procurement services for QC instruments and Production Equipments/Instruments

- Provide support in purchase of Laboatory glassware.
- Provide support in procurement of various equipments/instruments required for production and QC lab.
- Provide support in set up of QC and Microbiology Labs.
Support in implementation of Paperless Documentation (eBMR and eQMS LIMS etc.)
- Assessments of client requirements
- Implementation of electronic systems in compliance with 21 CFR.
- Verification of systems.
- Audits of electronic systems.

Recruitment and career counseling services

- Recruitment support for various technical/Non Technical positions.
- Counseling support to students and industry professionals for their growth in various streams.
GMP audit preparation for USFDA, MHRA, PIC/S, ANVISA, WHO-GMP etc
- GMP gap assessment and remediation of Quality systems, Manufacturing, laboratory and Engineering systems.
- Development of document management system.
- Development of entire Quality and GMP System.
- Perform Equipment, Utility and Facility Qualification.
- Perform process, analytical and cleaning validation
- Perform risk assessment of the system and facility.

